It has been approved as a prescription-only software-as-a-medical device in the US.
ResApp gets 510(k) clearance for mobile sleep apnoea test SleepCheck
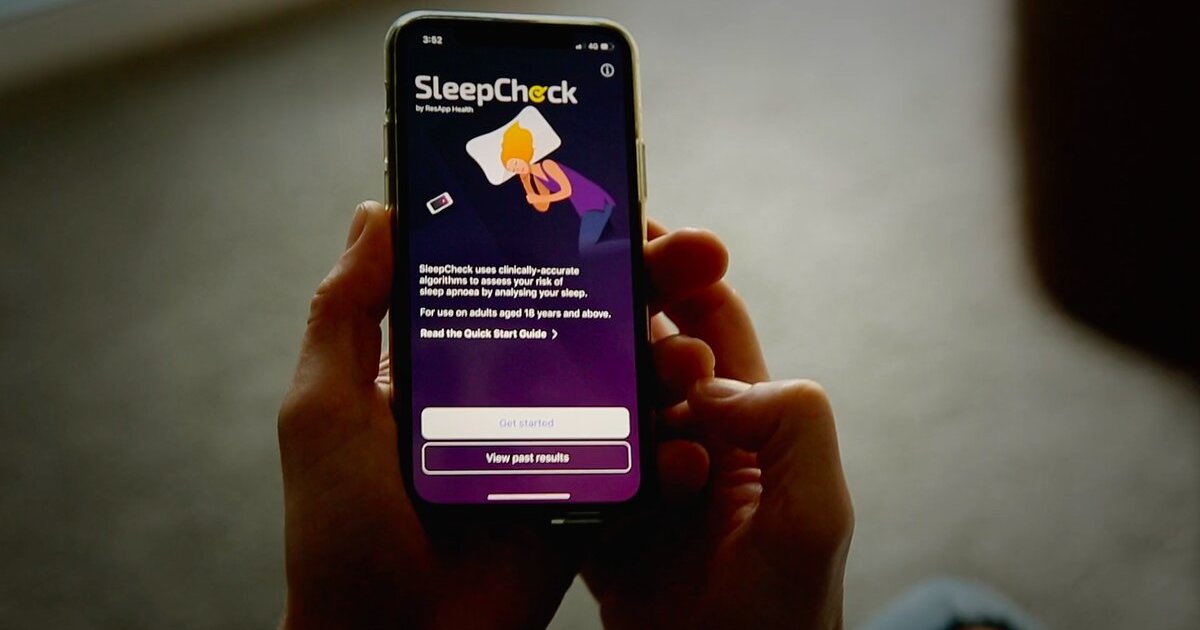

It has been approved as a prescription-only software-as-a-medical device in the US.